The revelation of new components is a significant area of logical investigation, stamping key achievements in science and physical science. These revelations have molded the cutting-edge comprehension of issues and the intermittent table of components.
The most common way of finding new components, especially those past uranium (transuranic components), has additionally prompted headways in atomic physical science and science.
Here is a point-by-point outline of the revelation of new components in logical history:
- Early Revelation of Components
In antiquated times, individuals utilized components like gold, copper, and silver without grasping their nuclear construction. In any case, as science advanced, components were efficiently recognised.
Hydrogen (1766): Henry Cavendish secluded hydrogen gas and remembered it as an unmistakable substance, referring to it as “inflammable air.” This is noticeable in the ID of the primary component in the cutting-edge sense.
Oxygen (1774): Joseph Priestley and Carl Wilhelm Scheele autonomously found oxygen, with Antoine Lavoisier remembering it as a component fundamental for ignition and breath.
Nitrogen (1772): Daniel Rutherford disengaged nitrogen, or “azote,” and perceived that it made up a huge piece of the World’s air.
Chlorine (1774): Found via Carl Wilhelm Scheele, chlorine was subsequently recognized as a substance component instead of a compound.
- The Intermittent Table and Mendeleev’s Expectations
The occasional table was a significant leap forward in sorting out components as per their nuclear properties.
Dmitri Mendeleev (1869): Mendeleev’s occasional table organized known components by nuclear mass, uncovering that components had repeating properties in gatherings. This association anticipated the presence of unseen components, and a few of these were found not long after Mendeleev’s work.
The expectations assisted researchers with zeroing in on missing components, prompting the ID of gallium (Ga), scandium (Sc), and germanium (Ge), which fit impeccably into Mendeleev’s table.
- nineteenth to Mid-twentieth century: Revelation of Components through Trial and Error
With the progression of exploratory procedures, more components were found in the late nineteenth and mid-twentieth centuries.
Radium (1898): Found by Marie and Pierre Curie, radium was a vital component in the investigation of radioactivity. Its disclosure prompted the comprehension of atomic rot processes.
Polonium (1898): Likewise found by the Curies, polonium was named after Poland and was critical in the investigation of atomic responses.
Thorium (1898): Found by Jöns Jakob Berzelius, thorium was the main component distinguished in the actinide series, which was later pivotal in atomic physical science.
- The Revelation of Transuranic Components: Start of Atomic Science
The quest for components past uranium (components with nuclear numbers more noteworthy than 92) began in the mid-twentieth hundred years, with forward leaps in atomic science.
Neptunium (Np, 93) – 1940
Disclosure: Glenn T. Seaborg, Edwin McMillan, and others at the College of California, Berkeley, made neptunium by barraging uranium with neutrons. It was the main component to be incorporated past uranium.
Importance: This obviously the start of the actinide series, which incorporates numerous engineered components.
Plutonium (Pu, 94) – 1942
Disclosure: Following the revelation of neptunium, plutonium was made by a similar group, and it turned into a pivotal component in atomic responses.
Importance: Plutonium assumed a significant part in the improvement of thermal power and nuclear weapons.
Americium (Am, 95) – 1944
Revelation: Americium was combined by assaulting plutonium with neutrons. It was the first engineered component broadly utilized in quite a while, like in smoke alarms.
Importance: Americium was one of the main fake components utilized in useful customer items.
Curium (Cm, 96) – 1944
Revelation: Curium, named after Marie Curie, was made by assaulting plutonium with alpha particles. Its revelation advanced the investigation of radioactivity and the atomic construction of molecules.
Importance: Curium was utilized in logical examination and as an intensity source in space investigation.
- The twentieth 100 years and Superheavy Components
As molecule gas pedals progressed, researchers started to make heavier and heavier components by atomic combination.
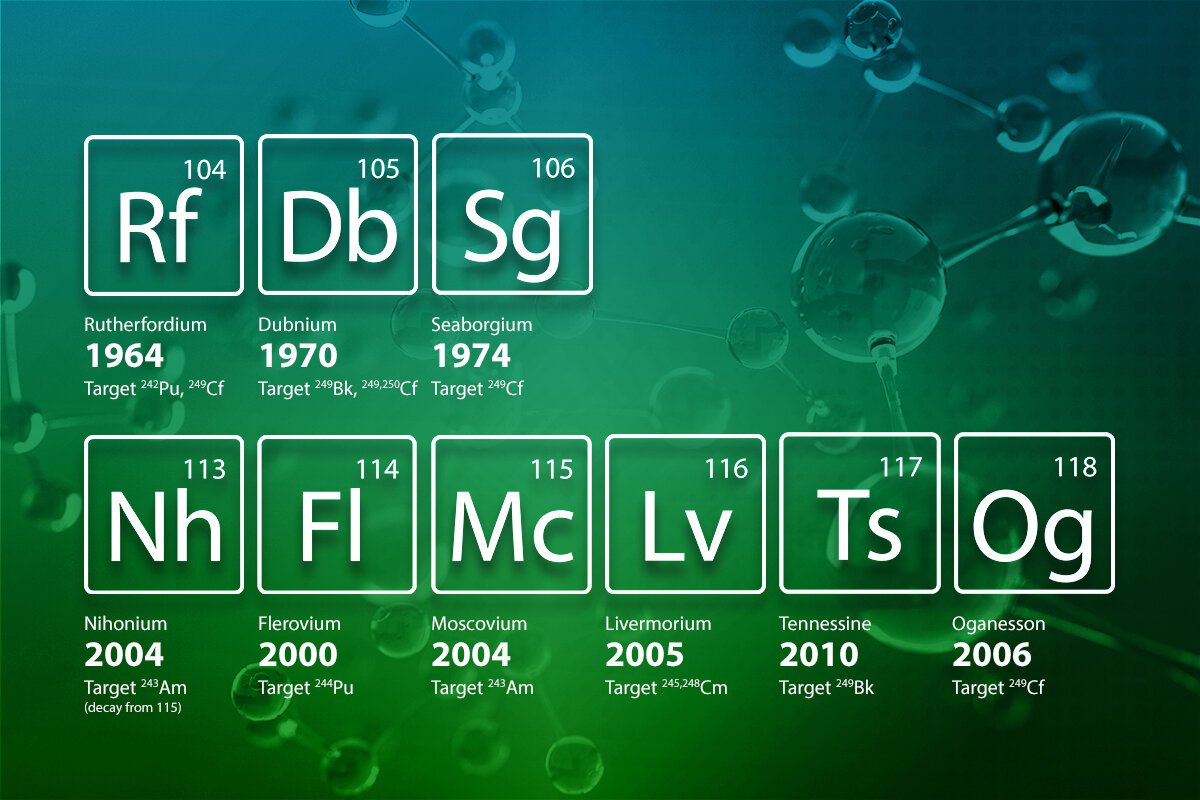
Rutherfordium (Rf, 104) – 1964
Disclosure: Rutherfordium was first orchestrated in 1964 by a group of researchers from the Joint Foundation for Atomic Exploration in Russia, and it was named to pay tribute to Ernest Rutherford, the dad of atomic physical science.
Importance: It was the primary component in period 7 of the occasional table.
Dubnium (Db, 105) – 1970s
Disclosure: Dubnium was blended by American and Russian groups, and it was named after Dubna, the Russian city where the component was first made.
Importance: This is noticeable in the fruitful production of another transuranic component.
Seaborgium (Sg, 106) – 1974
Disclosure: Seaborgium was named after Glenn T. Seaborg, an unmistakable atomic scientific expert who assumed a significant part in the disclosure of transuranic components.
Importance: This revelation further affirmed the presence of superheavy components in the occasional table.
Components 107-112:
Bohrium (Bh, 107), Hassium (Hs, 108), Meitnerium (Mt, 109), Darmstadtium (Ds, 110), Roentgenium (Rg, 111), and Copernicium (Cn, 112) were completely made by specialists utilizing atomic combination strategies, expanding upon crafted by before researchers.
These components, situated in periods 7 and 8, finished the filling of the seventh time of the occasional table.
- The Disclosure of Components 113-118: Current Advances
Nihonium (Nh, 113) – 2004
Disclosure: Nihonium was the primary component authoritatively found by Japanese researchers at the Riken lab in Japan. It was named after Japan (Nihon).
Importance: Nihonium’s revelation denoted the start of the investigation of the “f-block” components past the actinides.
Flerovium (Fl, 114) – 2011
Revelation: Made by Russian and American researchers, Flerovium was named after the Flerov Lab of Atomic Responses in Dubna, Russia.
Importance: Flerovium was a vital achievement in the quest for superheavy components.
Moscovium (Mc, 115) (2016)
Revelation: Named after Moscow, Moscovium was founded in a coordinated effort between Russia and the US.
Importance: Its creation shows the fruitful combination of one more superheavy component in the occasional table.
Livermorium (Lv, 116) – 2012
Disclosure: Livermorium was made by the Joint Foundation for Atomic Exploration (Russia) and Lawrence Livermore Public Lab (USA), named after the research facility.
Importance: Livermorium’s disclosure further finished the filling of the seventh time frame.
Tennessine (Ts, 117) – 2010
Revelation: Tennessine was named after the territory of Tennessee and was integrated by Russian and American groups.
Importance: Tennessine is essential for the halogen bunch, finishing the halogen series in the seventh period.
Oganesson (Og, 118) – 2002
Disclosure: The revelation of Oganesson denoted the last component in the occasional table. It was named after Yuri Oganessian, a conspicuous Russian researcher.
Importance: Oganesson is a component in the respectable gas gathering, and it is the heaviest component presently known. - The Quest for Component 119 and then some
Researchers are currently centered around the quest for component 119 and then some, which would require progressed molecule gas pedals and new techniques for blending superheavy components.
Hypothetical models recommend that these superheavy components might display strange properties, conceivably settling in specific designs, making a potential “island of solidness.”
The combination of these components is a significant wilderness of current atomic science and physical science, with expected ramifications for grasping the central idea of the issue.
conclusion
The disclosure of new components has been a basic piece of grasping the design of the issue. From the primary components known to old civic establishments to the cutting-edge formation of superheavy components, every disclosure extends the intermittent table and brings new experiences into atomic material science and science.
The difficulties of blending new components, especially those past uranium, require progressed procedures in atomic combination and molecule gas pedals. As examination advances, almost certainly, more superheavy components will be found, and new applications for these components might arise in fields like materials science, energy, and even medication.